UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 18, 2020
C4 THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
Delaware |
001-39567 |
47-5617627 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
|
490 Arsenal Way, Suite 200 Watertown, MA |
|
02472 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (617) 231-0700
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
Common Stock, $0.0001 par value per share |
|
CCCC |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure
On November 18, 2020, C4 Therapeutics, Inc. (the “Company”) posted an investor presentation to its website at https://ir.c4therapeutics.com/events-presentations. A copy of the investor presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed subject to the requirements of amended Item 10 of Regulation S-K, nor shall it be deemed incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing. The furnishing of this information hereby shall not be deemed an admission as to the materiality of any such information.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits. The exhibits shall be deemed to be filed or furnished, depending on the relevant item requiring such exhibit, in accordance with the provisions of Item 601 of Regulation S-K (17 CFR 229.601) and Instruction B.2 to this form.
|
Exhibit Number |
|
Description |
|
99.1 |
|
Investor Presentation of the Company dated November 2020 (furnished herewith). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
C4 Therapeutics, Inc. |
|
|
|
|
|
|
|
Date: November 19, 2020 |
|
By: |
/s/ Andrew J. Hirsch |
|
|
|
|
Andrew J. Hirsch |
|
|
|
|
President and Chief Executive Officer |

Corporate Presentation November 2020 © 2020 C4 Therapeutics, Inc.

Forward-looking Statements and Intellectual Property Forward-looking Statements The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. Intellectual Property C4 Therapeutics, Inc. owns various registered and unregistered trademarks in the U.S. and overseas, including, without limitation, C4 THERAPEUTICS, TORPEDO, BIDAC and MONODAC. All trademarks or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. © 2020 C4 THERAPEUTICS, INC.

C4 Therapeutics’ Vision © 2020 C4 THERAPEUTICS, INC. Harnessing the body’s natural cellular quality control mechanisms to target and destroy disease-causing proteins Perfecting a novel discovery and validation platform to rapidly and cost-effectively bring transformative medicines to patients Pioneering protein degradation as a new class of medicine by:

Lead product candidate (CFT7455) exhibited complete regression of tumor in preclinical multiple myeloma mouse model Potency and speed of lead molecule results in cell death at very low doses IND filing for lead program expected by end of 2020 Enables quick and efficient advancement of programs from target identification to candidate development stage Proprietary tools to design, assess and predict degrader activity Degraders optimized for speed and potency Deep investment in Cereblon Four programs expected to be in the clinic by the end of 2022 MonoDACs and BiDACs Lead targets selected to min dev risk: Clinically validated targets Expectation of tolerability Opportunity for accelerated approval Have received $150M+ in non-dilutive capital, with $2B+ in unrealized potential milestones, plus additional potential royalties Degraders control protein levels by leveraging the cell’s natural “quality control” system Label harmful or disease-causing proteins for destruction rather than merely inhibiting their function Investment Highlights Robust Pipeline Differentiated and Proprietary Technology Platform Novel and Transformative Modality High-Value Partnerships Anchored by Lead Program Addressing Unmet Need © 2020 C4 THERAPEUTICS, INC.

Degradation Leverages Natural Cellular Machinery to Target Diseases Protein degraders harness the body's natural protein degradation machinery to target and destroy selected disease-causing proteins 3. “The Destroyer” – The Proteasome Labelled proteins are destroyed by the cell’s “recycling plant,” the proteasome C4 Therapeutics’ Degraders: The Match Makers that Let the Body Do the Heavy Lifting 2. “The Quality Control Inspector” – Cereblon Applies a “destroy me” label on the target proteins recruited by the C4T degrader 1. “The Match Maker” – C4T’s Degraders C4T’s degraders direct Cereblon (a clinically validated E3 ligase) to label a specific disease-causing target protein for degradation, allowing the body’s natural protein degradation process to destroy the protein and control the disease C4T’s Technology Natural Machinery Degrader Target Ligase Complex Cereblon © 2020 C4 THERAPEUTICS, INC.

Targeted Protein Degradation: An Integrated Approach for a Novel Modality C4 Therapeutics maintains a broad focus on overall catalytic degradation rather than a specific part of the degradation cycle, providing opportunities to drug the undruggable Degraders control protein levels by leveraging the cell’s natural “quality control” or “protein recycling” system through a process called ubiquitination Degraders induce targeted destruction of harmful or disease-causing proteins by tagging them for ubiquitination Tagged proteins are subsequently degraded by the proteasome A single degrader molecule participates in multiple rounds of targeted protein target degradation, maximizing both potency and efficiency Overview of Targeted Protein Degraders © 2020 C4 THERAPEUTICS, INC.

Protein Degradation is Fundamentally Different than Protein Inhibition Protein degraders allow for a more potent and durable pharmacological response at lower overall exposure levels than inhibitors Improved Potency Degraders are recycled and can engage multiple target proteins, resulting in improved activity against resistant proteins, greater depth of effect, and more durable outcomes Fast Response Rapid degradation of target leads to strong and prolonged biological response High Selectivity Degraders can leverage multiple layers of selectivity in cellular machinery Expansive Target Landscape Degraders can be designed to bind to any part of the protein and are not limited to the active site, like most small molecule inhibitors, which means that previously undruggable targets may be degraded Key Advantages of Efficient Catalysis © 2020 C4 THERAPEUTICS, INC.

TORPEDO Platform: Robust Drug Discovery and Higher Confidence in Clinical Outcomes Design Analyze Predict Computational method incorporates experimental data to identify top models Atomic-level degrader design utilized to improve selectivity and potency Cellular degradation data fitted using an enzymology framework Key parameters describe intrinsic degradation activity Universal modeling framework merges degradation activity with degrader exposure Robust predictions of depth and duration of in vivo target degradation at any dose HDX-MS Predicted ternary complexes Rapid delivery of potent drug candidates through informed and efficient drug discovery © 2020 C4 THERAPEUTICS, INC.

PK/PD Models Provide Robust Predictions Across the Lead Programs CFT-12521, 30 mg/kg dose iv, KIF-5B:RET Fusion model CFT7455, 1 mg/kg dose po, KI-KJ ALCL model CFT7503, 10 mg/kg dose po, Yamato Synovial Sarcoma model CFT-17977, 30 mg/kg dose po, A375 model © 2020 C4 THERAPEUTICS, INC. PK = pharmacokinetics; PD = pharmacodynamics

Cereblon E3 Ligase TORPEDO is Based on a Deep Focus on Cereblon, Rather than the Entire Set of Available Ligases Our TORPEDO platform has a diverse and rich toolkit of novel, structurally distinct Cereblon binders – small molecules that are suitable for clinical targeted protein degradation Invested heavily in rich toolkit of intellectual property with 14 structurally distinct Cereblon binders, giving C4T a competitive advantage Cereblon is expressed in all tissues and in all cellular compartments Cereblon, harnessed by lenalidomide, or IMiDs, is the only clinically validated ligase for targeted protein degradation All projects benefit from desirable properties offered by C4T’s Cereblon binders C4T’s binders enable drug discovery with enhanced oral bioavailability, solubility, permeability and stability © 2020 C4 THERAPEUTICS, INC.

Robust Pipeline With Four Clinical Programs Anticipated By End of 2022 Target selection emphasizes target validated, mitigated tolerability risks and opportunity for accelerated approval © 2020 C4 THERAPEUTICS, INC.

CFT7455: Clear Unmet Need Combined with Compelling Development Opportunity IKZF1/3 Mutations Strong Mechanistic Rationale Multiple myeloma (MM) and Non-Hodgkin lymphomas (NHLs) are dependent on IKZF1/3 Clear Unmet Need IKZF1/3 degraders are the backbone of MM treatment Relapsed / refractory MM remains an unmet medical need Approved IMiDs have limited activity in NHL, including mantle cell lymphoma (MCL) and peripheral T-cell lymphoma (PTCL) Defined Patient Population MM: ~32,270 cases/year (US); median 5-year overall survival (OS): 53.9% NHL: ~77,240 cases/year (US) - PTCL: ~4% of all NHLs (US); median 5-year OS: 20-32% - MCL: ~7% of all NHLs (US); median OS of 4-5 years Compelling Development Opportunity Opportunity to expand into early lines of MM therapy IKZF1 and IKZF3 are central to lymphoid cell differentiation and maintenance Potential Clinical Indications Multiple myeloma Other B-cell lymphomas – MCL, diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma PTCL represents unrealized path for development Additional indication line extension potential G-loop Source: NIH SEER Database, Primary Literature Consensus © 2020 C4 THERAPEUTICS, INC.

CFT7455 Is a Potent IKZF1/3 MonoDAC, as Demonstrated in Preclinical Studies CFT7455 Viability Profile CFT7455 Degradation Potency Catalytic activity results in potent degradation and activity © 2020 C4 THERAPEUTICS, INC.

CFT7455: Potent Efficacy, In Vivo, with Complete Regressions as Single Agent in MM Potency and speed of CFT7455 translates into better cell killing with lower doses achieving the same endpoint Improvement in oral bioavailability (i.e., half dose provides same exposure) Pharmacologic properties translate into 100x lower dose in vivo to get to same efficacy Differentiated pharmacologic profile may also enable intermittent daily dosing Deep and durable responses observed as single agent Comparable efficacy seen in models of PTCL, MCL and DLBCL C4T Compound Celgene Compound Key: Complete Regression in MM Model Pomalidomide, 3,000 µg/kg CC-92480, 300 µg/kg CC-92480, 1,000 µg/kg CFT7455, 30 µg/kg CFT7455, 10 µg/kg CFT7455, 3 µg/kg Vehicle © 2020 C4 THERAPEUTICS, INC.

Multiple Myeloma Xenograft Insensitive to Pomalidomide Responds to CFT7455 CFT7455 is active in RPMI-8226, relative to pomalidomide CFT7455 is active in RPMI-8226 after the tumor has progressed on pomalidomide treatment RPMI-8226 Tumor Volume (mm3) Days of Treatment Pomalidomide, 3,000 µg/kg Vehicle CFT7455, 30 µg/kg Day 21 Days of Treatment 2,500 0 500 1,000 1,500 2,000 RPMI-8226 Tumor Volume (mm3) © 2020 C4 THERAPEUTICS, INC.

(1) 28-day cycle / dose limiting toxicity (DLT) window (2) Combination therapy cohorts will open once each CFT7455 dose level has been cleared for safety Phase 1 Dose Escalation R/R Multiple Myeloma Monotherapy N =~30 Cohort B1: Monotherapy R/R Multiple Myeloma N =~12 Cohort C: Monotherapy Non-Hodgkin Lymphoma N =~12 Merkel Cell Lymphoma N=~20 Peripheral T-Cell Lymphoma N=~20 Cohort A: Monotherapy(1) R/R Multiple Myeloma (MM) & Non-Hodgkin Lymphoma (NHL) N =~6-12 Primary objectives are to characterize safety and tolerability and estimate anti-tumor activity, with key secondary objective to assess pharmacokinetics Trial design allows for three potential indications, each with opportunity for accelerated approval if expansion portions are successful Expansion stage doses will be at maximum tolerable dose (MTD) / recommended Phase 2 dose (RP2D) with different dosing strategy for MM vs. NHL due to historical lower tolerability for NHL and need for combination with dexamethasone in MM Proposed CFT7455 First-In-Human Trial Design Offers Potential for Accelerated Approval Phase 2 Expansion © 2020 C4 THERAPEUTICS, INC. Cohort B2: Combination with Dexamethasone(2) R/R Multiple Myeloma N =~12 R/R Multiple Myeloma with Dexamethasone N =~30

BAF subunit mutations are present in >20% of cancers BRD9 Degradation: Clear Clinical Opportunity BRD9/BAF Complex Alterations Summary Strong Mechanistic Rationale Bromodomain containing protein 9 (BRD9) is a BAF complex component and is non-essential in normal cells Synovial sarcoma is dependent on BRD9, which is caused by the oncogenic SS18-SSX translocation Clear Unmet Need Very limited benefit of treatments for metastatic synovial sarcoma or advanced synovial sarcoma – median survival ~18 months Defined Patient Population ~900 US yearly incidence of synovial sarcoma cases ~10% of all soft tissue sarcoma Compelling Development Opportunity Initial Target Population: Synovial Sarcoma, after 1L therapy failure Well defined path to registration in synovial sarcoma and metastatic population already under management at academic treatment centers Precedent for approval in uncontrolled study in second-line setting 100% of synovial sarcoma 100% of malignant rhabdoid tumors Potential in other emerging indications (e.g., acute myeloid lymphoma) Potential Clinical Indications Source: NIH SEER Database, Primary Literature Consensus © 2020 C4 THERAPEUTICS, INC.

Robust, Dose-Dependent Response in Yamato Xenograft Model Achieved with CFT8634* at Tolerable Dose Levels * CFT8634 is the purified enantiomer of CFT7503 (a racemic mixture) and is the drug candidate moving into clinical development Tolerated at all doses Dose responsive activity © 2020 C4 THERAPEUTICS, INC.

Phase 1 Dose Escalation Phase 2 Expansion CFT8634 MTD/RP2D Cohort B: CFT8634 Monotherapy Synovial Sarcoma N = 20 Cohort A: CFT8634 Monotherapy Synovial Sarcoma and SMARCB1 deleted solid tumors N =~18 Cohort C: CFT8634 Monotherapy SMARCB1 deleted tumors N = TBD CFT8634 First-in-Human Protocol Concept Schema © 2020 C4 THERAPEUTICS, INC.
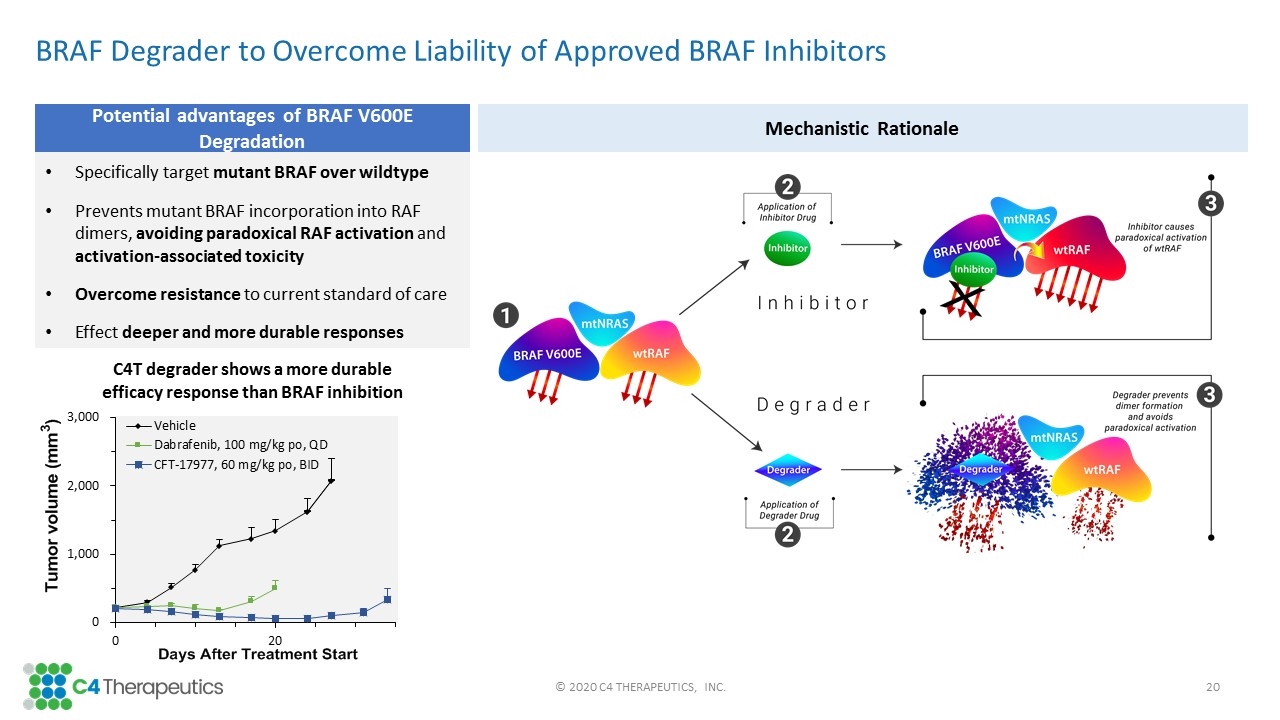
BRAF Degrader to Overcome Liability of Approved BRAF Inhibitors Specifically target mutant BRAF over wildtype Prevents mutant BRAF incorporation into RAF dimers, avoiding paradoxical RAF activation and activation-associated toxicity Overcome resistance to current standard of care Effect deeper and more durable responses Potential advantages of BRAF V600E Degradation Mechanistic Rationale C4T degrader shows a more durable efficacy response than BRAF inhibition © 2020 C4 THERAPEUTICS, INC.

RET Degradation May Significantly Improve Activity of Best-In-Class RET Inhibitors Overcome resistance to standard of care RET inhibitors Potential to effect deeper and more durable response due to advantages of degraders Potential advantages of RET Degradation Mechanistic Rationale C4T RET degrader is as effective as approved RET inhibitors © 2020 C4 THERAPEUTICS, INC.

Potential for Multiple Near-term Milestones Across Lead Programs Pro-Forma cash balance of $390M as of 9/30/20 provides runway into H2 2023 Note: Other undisclosed targets have potential milestones during this time frame; cash runway guidance as of 11/12/2020 IKZF1/3 (CFT7455) BRD9 (CFT8634) BRAF RET IND Enabling Study Initiation Phase 1 Initiation IND Submission Phase 1 Initiation Phase 1 Top-line Safety Data Phase 1 Top-line Efficacy Data Proof of Mechanism Top-Line Safety Data Phase 1 Initiation Phase 1 Initiation 2020 2021 2022 IND Submission © 2020 C4 THERAPEUTICS, INC.

© 2020 C4 THERAPEUTICS, INC.
