UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 7, 2021
C4 THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
Delaware |
001-39567 |
47-5617627 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
|
490 Arsenal Way, Suite 200 Watertown, MA |
|
02472 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (617) 231-0700
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
Common Stock, $0.0001 par value per share |
|
CCCC |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On June 7, 2021, C4 Therapeutics, Inc. (the “Company”) issued a press release entitled “C4 Therapeutics Presents Pre-clinical Data on CFT8919, A Selective Degrader of EGFR L858R, at Keystone Symposium on Targeted Protein Degradation.” The Company also posted a corporate presentation on CFT8919 pre-clinical data for its investor call on its website at https://ir.c4therapeutics.com/events-presentations. A copy of the press release and the corporate presentation are filed as Exhibits 99.1 and 99.2, respectively, to this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits. The exhibits shall be deemed to be filed or furnished, depending on the relevant item requiring such exhibit, in accordance with the provisions of Item 601 of Regulation S-K (17 CFR 229.601) and Instruction B.2 to this form.
|
Exhibit Number |
|
Description |
|
99.1 |
|
|
|
99.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
C4 Therapeutics, Inc. |
|
|
|
|
|
|
|
Date: June 7, 2021 |
|
By: |
/s/ William McKee |
|
|
|
|
William McKee |
|
|
|
|
Chief Financial Officer |
Exhibit 99.1
 C4 Therapeutics Presents Pre-clinical Data on CFT8919, A Selective Degrader of EGFR L858R, at Keystone Symposium on Targeted Protein Degradation
C4 Therapeutics Presents Pre-clinical Data on CFT8919, A Selective Degrader of EGFR L858R, at Keystone Symposium on Targeted Protein Degradation
|
|
– |
CFT8919 Induces Tumor Regression in Pre-clinical Models Resistant to First- and Third-generation EGFR Inhibitors – |
|
|
– |
CFT8919 Demonstrates Intracranial Activity Pre-clinically, Indicating the Potential to be Effective Against CNS Metastases |
|
|
– |
Pre-clinical Data Support Plans to Advance CFT8919 to Clinical Development with IND Submission Expected in mid-2022 and Clinical Trial Initiation Expected by YE 2022 – |
|
|
– |
Conference Call and Webcast at 8:00 am ET Today– |
WATERTOWN, Mass., June 7, 2021 (GLOBE NEWSWIRE) – C4 Therapeutics, Inc. (C4T) (Nasdaq: CCCC), a biopharmaceutical company pioneering a new class of small-molecule medicines that selectively destroy disease-causing proteins through degradation, today presented new pre-clinical data on CFT8919, a novel mutant-selective degrader of epidermal growth factor receptor (EGFR) in non-small cell lung cancer (NSCLC) at the Keystone Symposium on Targeted Protein Degradation. The poster presentation shares pre-clinical data that suggests CFT8919 may be active as single agent in patients with resistance to EGFR inhibitors due to secondary mutations in EGFR, including T790M and C797S, as well as in the front-line setting with the potential to avoid the emergence of resistance-causing secondary EGFR mutations seen with currently approved EGFR inhibitors.
“We are excited to share strong preclinical data that establishes CFT8919 as a potent and selective degrader of EGFR L858R, a mutation responsible for more than a third of mutant EGFR lung cancer diagnoses. Patients with this mutation are commonly treated with approved EGFR inhibitor therapies, but often have worse clinical outcomes than individuals diagnosed with other driver mutations such as Exon19del,” said Adam Crystal, M.D., Ph.D., chief medical officer of C4 Therapeutics. “Across our portfolio, we see the potential for targeted protein degradation to transform patient care. We believe our decision to advance CFT8919 recognizes promising early data that indicate CFT8919 may have the potential to treat patients who develop resistance to first-line EGFR inhibitors as well as a path to inclusion in front-line therapeutic regimens. We look forward to learning more about CFT8919 as we advance the program into IND-enabling studies and initiate the Phase 1 clinical trial in 2022.”
Summary of CFT8919 Pre-clinical Results
C4T conducted in vitro and in vivo studies that show CFT8919 is a potent and highly selective orally bioavailable degrader of EGFR L858R with broad coverage of on-target resistance mutations as well as intracranial activity. Notable observations include:
|
|
• |
In cancer cell lines, CFT8919 induces degradation of EGFR L858R at low nanomolar concentrations while no degradation of wild type is induced up to 10 µM. |
|
|
• |
CFT8919 demonstrates equipotent activity against EGFR mutations resistant to EGFR inhibition, including L858R-C797S, L858R-T790M, and L858R-T790M-C797S compared to L858R single mutation in Ba/F3 cell models in vitro. |
|
|
• |
Kinome profiling and global proteomic evaluation did not identify any significant off-target activity of CFT8919. CFT8919 does not induce degradation of known cereblon neo-substrates SALL4 or GSPT1, indicating that the potential associated toxicities will not be liabilities. |
Additionally, in vivo data demonstrates the following:
|
|
• |
CFT8919 degrades and inhibits mutant EGFR in tumors upon oral administration. In the NCI-H1975 EGFR-L858R-T790M xenograft model, after a single oral dose of CFT8919, up to 85 percent of mutant EGFR was degraded in tumors accompanied with near-complete inhibition of phospho-EGFR. |
|
|
o |
In this model, twice-daily oral administration of CFT8919 showed dose-dependent anti-tumor activity and regressions comparable to osimertinib. |
|
|
• |
In BaF3 allograft model expressing EGRF-L858R-T790M-C797S, CFT8919 demonstrates anti-tumor activity resulting in tumor regression, while osimertinib is inactive. |
|
|
• |
CFT8919 demonstrates rapid and significant reductions in tumor burden in the H1975-LUC (EGFR L858R-T790M) brain metastasis model after oral dosing, indicating its potential to be active in the central nervous system. |
This promising pre-clinical data supports the Company’s decision to advance CFT8919 into investigational new drug (IND)-enabling studies this year. C4T anticipates filing an IND for CFT8919 by mid-2022, with the goal to initiate a Phase 1 clinical trial by year-end 2022.
C4T will host an investor event and webcast today, June 7, 2021, at 8 am E.T. to further discuss the pre-clinical data. Details of this event are included below.
In addition, the poster presentation from the Keystone Symposium on Targeted Protein Degradation will be archived on the “Scientific Publications” page in the Investors section of the Company’s website, located at www.c4therapeutics.com.
Investor Event and Webcast Information
C4T will host a live webcast today, Monday, June 7, 2021, at 8:00 a.m. E.T. to discuss the CFT8919 data presented at the Keystone Symposium. The webcast can be accessed through the Events and Presentations page on the Investors section of C4T’s website at www.c4therapeutics.com. A replay of the webcast will be available on C4T’s website for 30 days following the event.
About C4 Therapeutics
C4 Therapeutics (C4T) is a biopharmaceutical company focused on harnessing the body’s natural regulation of protein levels to develop novel therapeutic candidates to target and destroy disease-causing proteins for the treatment of cancer and other diseases. This targeted protein degradation approach offers advantages over traditional therapies, including the potential to treat a wider range of diseases, reduce drug resistance, achieve higher potency, and decrease side effects through greater selectivity. To learn more about C4 Therapeutics, visit www.c4therapeutics.com.
Forward-Looking Statements
This press release contains “forward-looking statements” of C4 Therapeutics, Inc. within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements may include, but may not be limited to, express or implied statements regarding our ability to develop potential therapies for patients; the design and potential efficacy of our therapeutic approaches; the predictive capability of our TORPEDO™ platform in the development of novel, selective, orally bioavailable degraders; the potential timing, design and advancement of our preclinical studies and clinical trials, including the potential timing for regulatory submissions and authorization related to clinical trials; our ability and the potential to successfully manufacture and supply our product candidates for clinical trials; our ability to replicate results achieved in our preclinical studies or clinical trials in any future studies or trials; our current resources and cash runway; regulatory developments or approvals in the United States and foreign countries; and upcoming events that C4T will participate in. Any forward-looking statements in this press
release are based on management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: uncertainties related to the initiation, timing, advancement and conduct of preclinical and clinical studies and other development requirements for our product candidates; the risk that any one or more of our product candidates will cost more to develop or may not be successfully developed and commercialized; and the risk that the results of preclinical studies and/or clinical trials will or will not be predictive of future results in connection with future studies or trials. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in C4 Therapeutics’ most recent Annual Report on Form 10-K and/or Quarterly Report on Form 10-Q, as filed with the Securities and Exchange Commission. All information in this press release is as of the date of the release, and C4 Therapeutics undertakes no duty to update this information unless required by law.
Investor Contact:
Kendra Adams
SVP, Communications & Investor Relations
Kendra.Adams@c4therapeutics.com
Media Contact:
Loraine Spreen
Director, Corporate Communications & Patient Advocacy
LSpreen@c4therapeutics.com

CFT8919 Pre-Clinical Data Investor Call June 7, 2021 Exhibit 99.2

Forward-looking Statements The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. Intellectual Property C4 Therapeutics, Inc. owns various registered and unregistered trademarks in the U.S. and internationally, including, without limitation, C4 THERAPEUTICS, our housemark logo, the name of our TORPEDO platform, and the names of our BIDAC and MONODAC degrader products. All trademarks or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. Forward-looking Statements and Intellectual Property 2

Today’s Agenda 3

What You Will Hear Today 4 CFT8919 is an orally bioavailable, selective, allosteric degrader of EGFR L858R Active in vitro and in vivo in models with secondary EGFR mutations Demonstrates intracranial activity indicating potential to prevent or treat brain metastases in patients with EGFR L858R-driven tumors 25-45% of mutant EGFR NSCLC is driven by L858R activating mutation; these patients are not adequately addressed with current EGFR therapies Pre-clinical data suggests CFT8919 has path to registration in EGFR patients who develop resistance to osimertinib Sources: Li, K et al. Oncol Rep 37, 1347–1358 (2017);Jin Y. et al. Scientific Reports 6:31636 (2016); Soria, J.-C. et al. NEJM 378, 113–125 (2018)

TORPEDO Platform Has Delivered a Robust Degrader Pipeline; Four Clinical Programs Expected by End of 2022 Nine Additional Undisclosed Collaborator Programs in Discovery 5
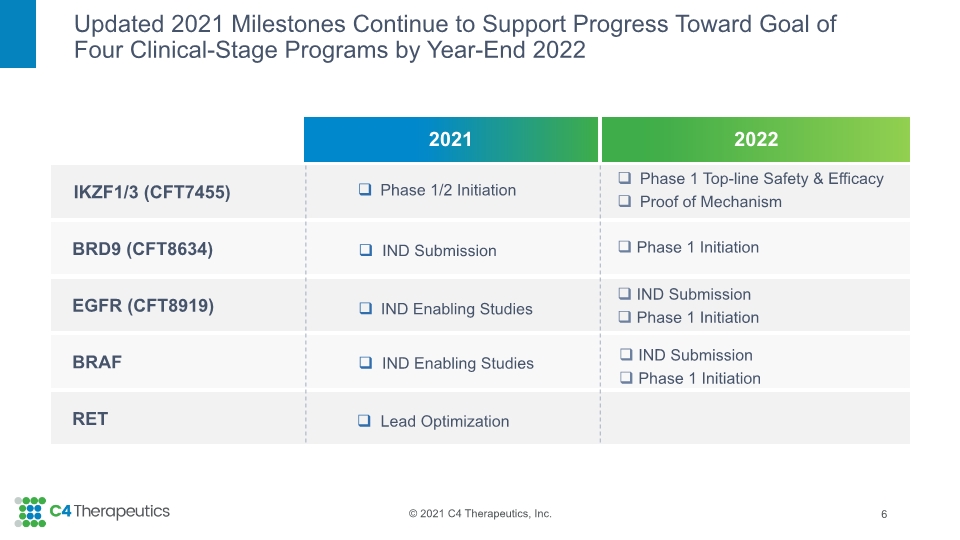
Updated 2021 Milestones Continue to Support Progress Toward Goal of Four Clinical-Stage Programs by Year-End 2022 6 Phase 1/2 Initiation IND Submission Lead Optimization IND Enabling Studies IND Enabling Studies Phase 1 Top-line Safety & Efficacy Proof of Mechanism Phase 1 Initiation IND Submission Phase 1 Initiation IND Submission Phase 1 Initiation

Preclinical Evaluation of CFT8919 as a Mutant Selective Degrader of EGFR with L858R Activating Mutations for the Treatment of Non-Small Cell Lung Cancer

Mutations in EGFR Drive Oncogenesis and Resistance in Non-Small Cell Lung Cancer 8 10-15% of Non-Small Cell Lung Cancer has Mutant EGFR 30-40% of Mutant EGFR NSCLC Patients will Develop Brain Metastases 25-45% of Mutant EGFR NSCLC is Driven by L858R Activating Mutation This rises to nearly 40% in Asian population CNS activity desirable to be competitive Sources: Zhang, Y.-L. et al. Oncotarget 7, 78985–78993 (2016); Li, K et al. Oncol Rep 37, 1347–1358 (2017); Shin, D.-Y. et al. J Thorac Oncol 9, 195–199 (2014); Rangachari, D. et al. Lung Cancer 88, 108-111 (2015); Jin Y. et al. Scientific Reports 6:31636 (2016); Soria, J.-C. et al. NEJM 378, 113–125 (2018) Patients with L858R have inferior clinical outcome

L858R mutation predicts less durable response to EGFR inhibitors No evidence that L858R is a more aggressive disease Despite Three Generations of Approved EGFR Inhibitors, L858R Patients Have Poorer Prognosis L858R Patients are Underserved by Current EGFR Inhibitor Therapies 9 Source: Soria, J.-C. et al. New Engl J Medicine 378, 113–125 (2018)

CFT8919 Selectively Targets EGFR-L858R in Human Cancer Cell Lines and is Not Impacted by EGFR T790M or C797S EGFR Degradation Phospho-EGFR Inhibition Degradation and Inhibition Potency 10 C B A

CFT8919 is Active in Ba/F3 Models Expressing Secondary Mutations Resistant to Approved EGFR Inhibitors Viability of Ba/F3 Cells Expressing the Indicated EGFR Variant Ba/F3 Cell Growth Inhibition Potency 11 B A
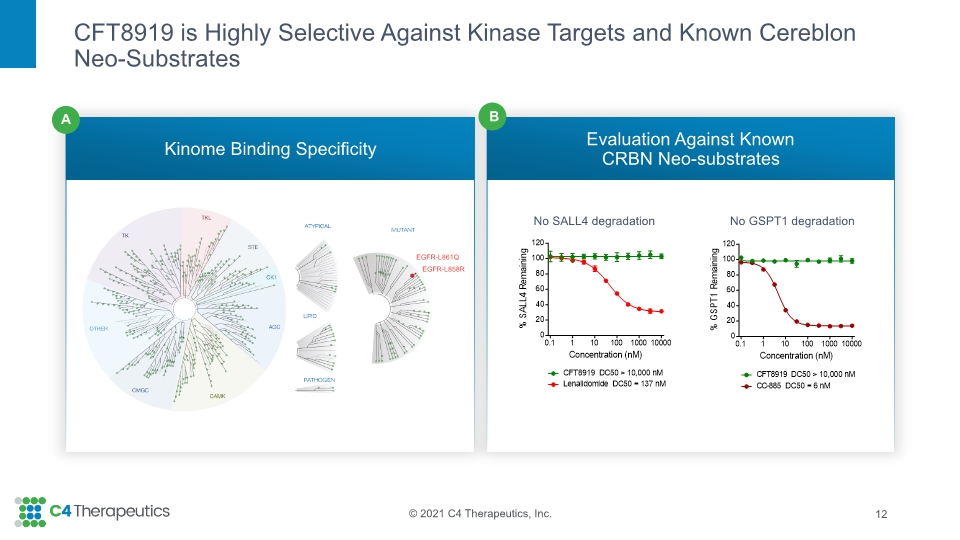
CFT8919 is Highly Selective Against Kinase Targets and Known Cereblon Neo-Substrates Kinome Binding Specificity Evaluation Against Known CRBN Neo-substrates 12 No SALL4 degradation No GSPT1 degradation B A
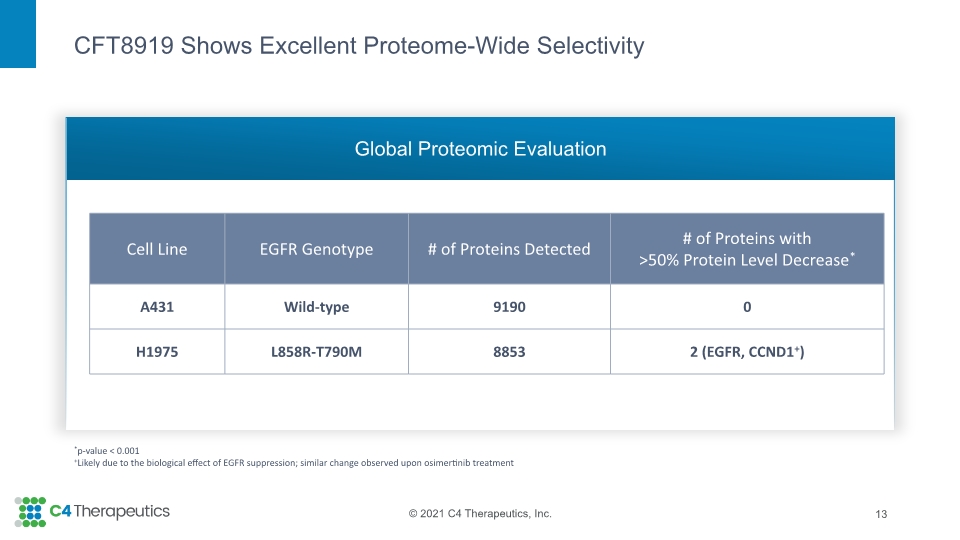
CFT8919 Shows Excellent Proteome-Wide Selectivity Global Proteomic Evaluation 13 *p-value < 0.001 +Likely due to the biological effect of EGFR suppression; similar change observed upon osimertinib treatment

CFT8919 Degrades and Inhibits Mutant EGFR in Tumors Upon Oral Administration 14 Tumor PD in H1975 EGFR-L858R-T790M xenograft model

CFT8919 Induces Tumor Regression in Mouse Models Resistant to First and Third-Generation EGFR Inhibitors 15 3rd-Generation EGFRi Resistant Ba/F3 (L858R-T790M-C797S) Allograft 1st-Generation EGFRi Resistant H1975 (L858R-T790M) Xenograft

CFT8919 Demonstrates Activity in H1975-LUC (EGFR-L858R-T790M) Brain Metastasis Model Mean Plasma & Tumor Concentration In vivo Efficacy In vivo Body Weight Change 16 Plasma clearance t1/2 = 3.1 hrs

Active in vitro and in vivo in models with secondary mutations (such as T790M, C797S, T790M-C797S) that cause acquired resistance to 1st-, 2nd-, and 3rd-generation EGFR inhibitors Demonstrates intracranial activity indicating potential to prevent or treat brain metastases in patients with EGFR L858R-driven tumors Clinical evaluation is warranted in patients with EGFR L858R driven NSCLC who have progressed on prior EGFR inhibitors By binding to an allosteric EGFR site, CFT8919 may combine with approved EGFR inhibitors which bind to the EGFR active site Pre-clinical profile highlight potential for single agent activity in the front-line setting CFT8919 is a Potent, Oral, Allosteric, Mutant-selective Degrader of EGFR L858R 17 IND Submission Expected mid-2022 with Potential Phase 1 Trial Initiation by YE 2022

Q&A Session

Thank You
